All you need is one simple blood test.
The Power of Early Cancer Detection – Made Simple
Know within 3 weeks if you have cancer, and what to do about it.
The LRNA Liquid Biopsy detects cancer earlier than any other test – across all known cancer-related genes and markers.
All you need is one simple blood test.
The Power of Early Cancer Detection – Made Simple
Know within 3 weeks if you have cancer, and what to do about it.
The LRNA Liquid Biopsy detects cancer earlier than any other test – across all known cancer-related genes and markers.
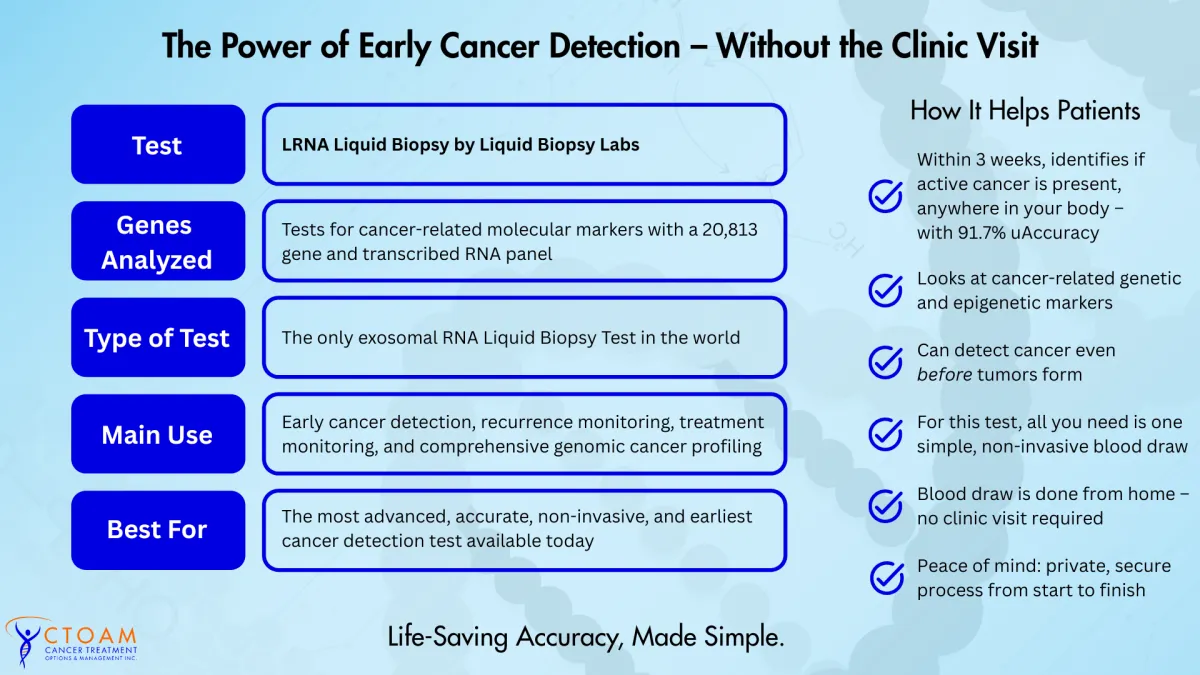
The earliest, fastest, cancer detection – within 3 weeks – without the clinic visit.
Tests for cancer-related molecular markers, across all types of solid and hematological malignancies.
LRNA Liquid Biopsy
Life-saving accuracy, made simple.
STEP 1: You can order the test yourself – your doctor is not needed.
STEP 2: A nurse comes to your home – you do not need to travel.
STEP 3: You receive results within 3 weeks – across all known cancer-related mutation and all potential treatment options worldwide!
Accepted at Leading International Oncology Congresses
We are pleased to share that CTOAM’s Liquid RNA abstract was selected at ASCO GI 2026 conference, in San Francisco, California, January 2026.
See the media presentation and abstract:
''Clinical performance of liquid RNA (L-RNA) biopsy in a gastrointestinal cancer segment.’’
CTOAM's Liquid RNA abstract has also been selected for presentation at the ESMO Targeted Anticancer Therapies Congress 2026.
Who is This Test For?
The LRNA Liquid Biopsy is Designed for You, If...
You are wondering if you have cancer and want to know for sure.
You have been diagnosed with cancer and want to confirm the risk of tumor cell activity.
You want to gain more information about the staging of your cancer, level of aggression, and treatment options.
You are currently on treatment and want to monitor how well the treatment is working.
You have been told your cancer is gone and want to confirm there is no risk of recurrence.
You are currently in remission and want to check for recurrence.
Early Cancer Detection, Without the Clinic Visit
Know within 3 Weeks if You Have Active Cancer.
Long wait times for diagnostics can mean weeks or even months before knowing for sure – which can be stressful and dangerous if cancer is present.
Delays in diagnosis can lead to more advanced disease and fewer treatment options.
Limited genetic testing in standard care means that even when you do receive a diagnosis, you don’t have enough information to know all of your treatment management options for specific mutation driven cancers.
Stay Home. Stay Informed. Stay Ahead.
The LRNA Liquid Biopsy closes this gap by providing you with fast, definitive answers – within just 3 weeks, with just one simple blood test.
This test focuses on just under 400 molecular features associated with early cancer transformation and includes 21,413 other markers associated with a variety of conditions.
Are You Wondering if You Have Cancer (or Recurrence)?
Early Answers, Right Where You Are.
Early Detection, Now at Your Convenience
What is So Special About This Test?
Primary Benefits of Using Exosomes in L-RNA for Cancer Diagnosis
The LRNA Liquid Biopsy is the most thorough and accurate liquid biopsy, using blood, for cancer detection and monitoring, using exosomal RNA expression, in the world today.
Tests for cancer-related molecular markers, including the latest genetic and epigenetic markers, with 95% accuracy.
Can detect cancer in the body before actual tumors have formed, across all known cancer-related genes and markers.
Validated across all types of solid and hematological malignancies, making it the most effective early cancer detection and treatment monitoring test.
Used for five distinct purposes:
Early detection of cancer and cancer recurrence
Screening where there is a family history of cancer
Identification of molecular targeted therapy treatment options
Identification of personalized dietary, nutraceutical, and integrative therapy options
Highly accurate cancer treatment efficacy monitoring
Have Questions?
Join our Live, Daily Drop-in Zoom Calls
Join our live, daily drop-in Zoom calls to get immediate answers from our co-founder & cancer care advocacy specialist, Michelle Morand.
Weekdays Monday to Friday from 9:30am to 10:30am PST (12:30pm to 1:30pm PST).
The Next Generation of Cancer Testing
All of the Answers, Without the Clinic Visit
Were you hoping to get a private PET-CT scan, only to find out you're not eligible?
In Canada, access to a private PET-CT scan typically requires one of the following:
– a confirmed cancer diagnosis;
– a pathology report showing cancer; or
– a CT or MRI report with findings labeled as "suspicious."
Or maybe you're exploring your options but are concerned about the radiation exposure from a PET-CT scan.
In either case, the LRNA Liquid Biopsy can be used as a minimally invasive, radiation-free way to determine when to do a PET-CT scan and to help support cancer-related decisions without relying solely on a PET-CT scan.
Shows signs of active cancer – BEFORE tumors have formed

Highly detailed, non-invasive, and simple

Shows level of aggression in tumors

Convenient – only one blood draw, arranged at your home

Fast results within 4 weeks, from start to finish
Next-generation sequencing at the highest level
Looks at over 20,813 molecular markers and genes

Provides in-depth data on your cancer's molecular features
The World's Most Advanced Liquid Biopsy-based RNA Expression Test (Using Blood):
Why This Matters
From Uncertainty to Clarity, Without the Clinic Visit
The LRNA Liquid Biopsy (using blood) only tests for active cancer cells.
Other cancer-related liquid biopsies use circulating tumor-DNA (ct-DNA).
None of the other ctDNA tests can differentiate between dead cells and active cancer cells.
This is why the accuracy rate of ct-DNA-based tests is so low.
This is also why you have to wait months after treatment and surgeries to use their tests: They can’t differentiate cells that are dead from treatment and cells that are live and still indicative of active disease.
Time Saved Could Mean Life Saved
Why Patients are Seeking Options Beyond the Public Healthcare System
When It Comes to Cancer, Every Week Counts
In this educational video, you'll see Precision Cancer Medicine Educator & Advocate, Michelle Morand, explain why wait times in Canada are currently out of control and creating harmful consequences for patients across the country.
One of our primary aims at CTOAM and Liquid Biopsy Labs is to provide patients in Canada with better options for detecting cancer early and getting an accurate diagnosis as soon as possible – because this is truly the very best thing you can do to increase your chances of treatment success and long-term remission.
Faster Answers. Better Outcomes
The Earlier You Are Diagnosed, the Greater Chance of Treatment Success
When it comes to cancer, time is critical. Early detection greatly increases the chances of successful treatment and long-term remission.
One LRNA Liquid Biopsy is all it takes to know whether cancer is present – with 95% accuracy – and if so, what the molecular profile of your cancer is.
Standard diagnostics like CT scans are too slow and often produce faulty results. They require at least two separate scans for comparison, often spaced months apart, plus additional waiting time for results – and often produce a false positive or false negative.
The World Health Organization Agrees:
"Early diagnosis leads to higher survival and less morbidity; delays mean lower survival, more treatment problems, and higher costs."
The LRNA Clarifies Unclear Image Results
Imaging only sees down to 0.5 cm and lack sufficient detail. This leads many imaging tools result in a “watch and wait” protocol for the patient – in part due to insufficient detail and precision in the image itself, and in part because many imaging tools (including CT scans) rely on comparison to determine results, meaning months of waiting between scans.
The LRNA liquid biopsy helps to clarify unclear imaging results, nearly immediately, with exceptional precision.
Survival Rates According to Stage of Cancer
The following chart shows the survival rates from a Canadian study on national stage-specific 5-year net survival rates (2010–2017, Canada excl. QC).
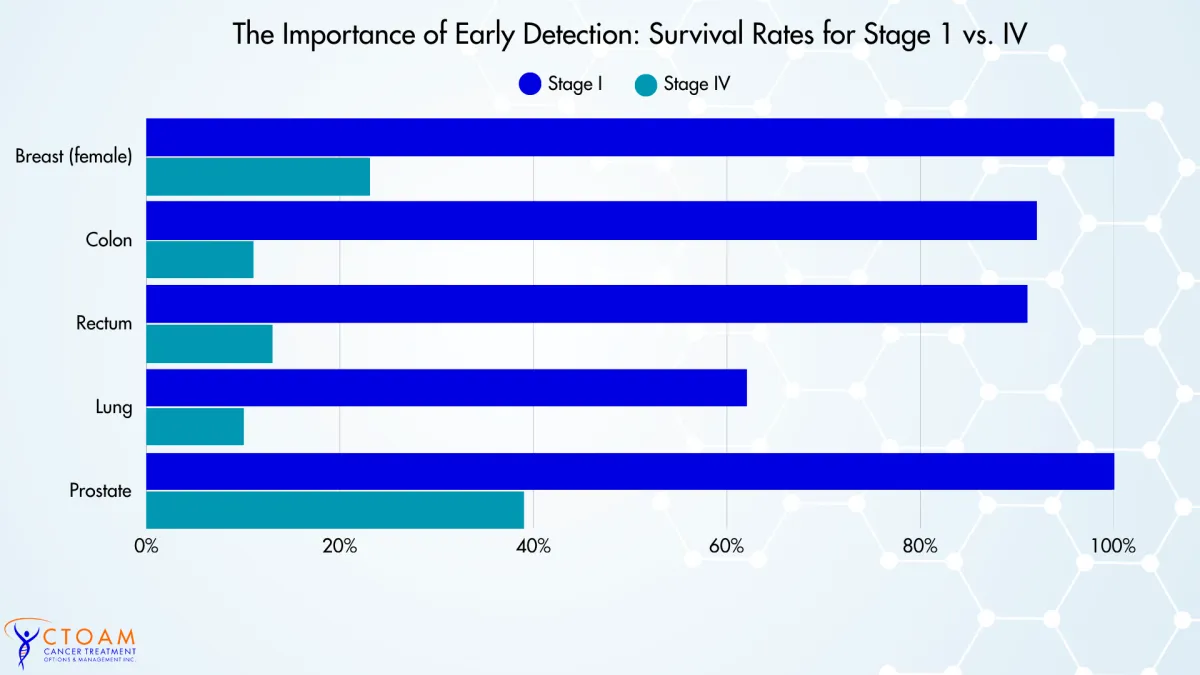
This study shows that early detection makes a dramatic difference in outcomes. Across cancers, 5-year survival is generally over 92% when found early, but falls steeply with advancing stage.
The Problem with Wait Times in Canada
Patients Don't Have Time to Wait. Every Week Matters.
Canada is facing a nationwide shortage of doctors and nurses, creating significant delays in diagnosis.
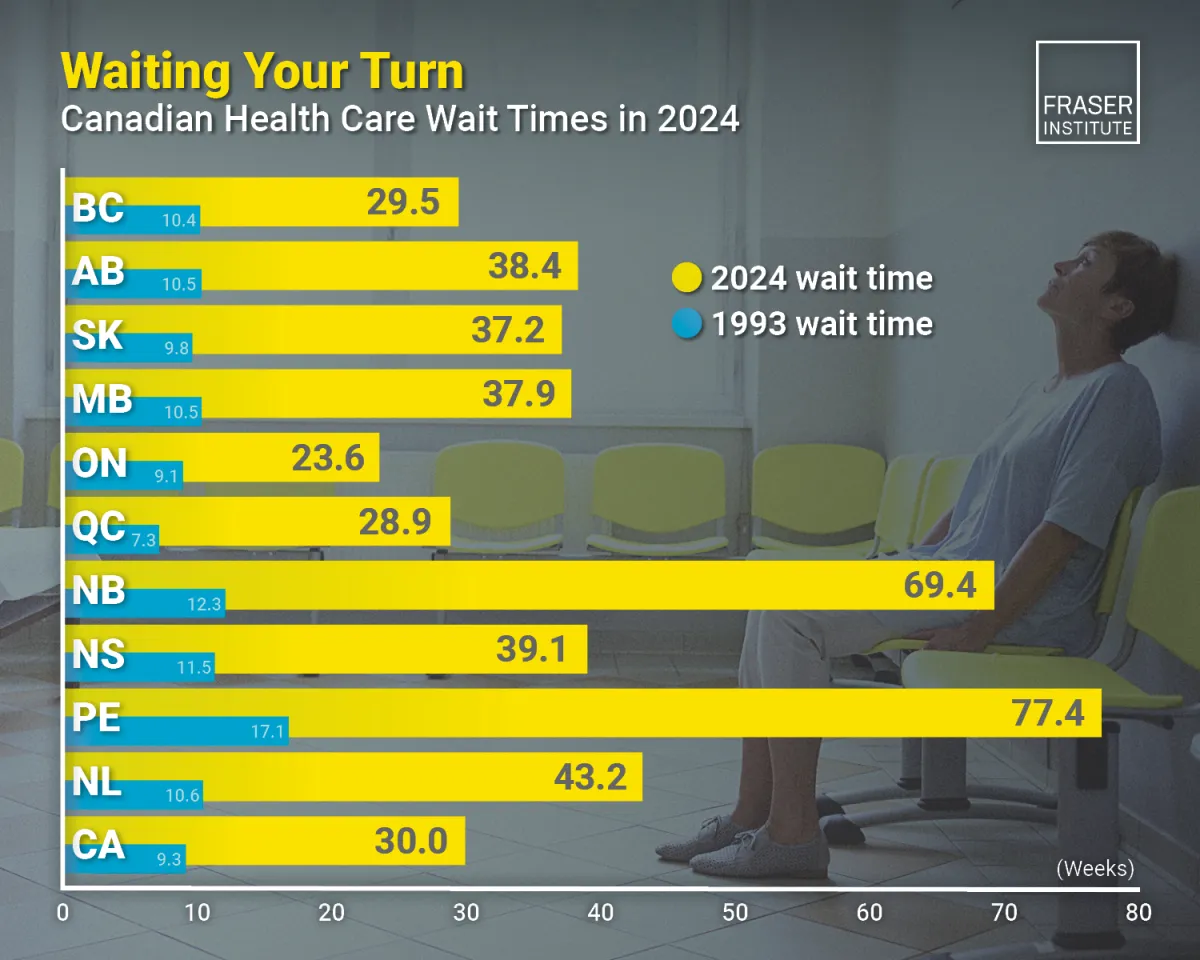
Canada's Doctor Shortage is Causing Delays in Diagnosis
Canada is facing a nationwide shortage of doctors and nurses, creating significant delays in diagnosis.
Patients across the country are often forced to wait many months – sometimes years – for an accurate cancer diagnosis.
Long diagnostic wait times can mean weeks or months of uncertainty, which is both stressful and dangerous if cancer is present.
Delayed diagnosis increases the risk of more advanced disease and fewer treatment options.
The Right Diagnosis & Treatment – Without the Wait
Get an Accurate Cancer Diagnosis in Three Weeks
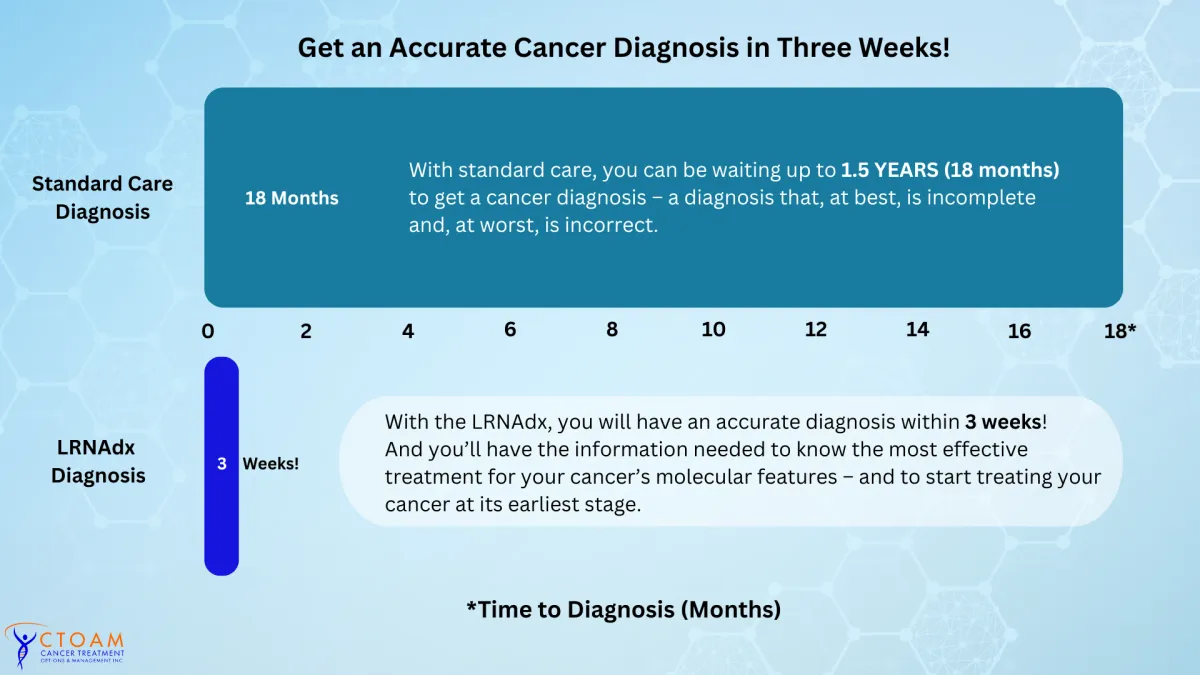
Speed and Accuracy Determine a Patient's Success
In standard care, it can take up to 18 months get a diagnosis...and, even then, the diagnose is incomplete, lacking the detail needed to identify all possible treatment options – or, in the worst case, the diagnosis is outright inaccurate.
With the LRNA, you will receive an accurate diagnosis, and critical information about the molecular features of your cancer, within 3 weeks!
And, if you would like to have further support from our team, we can move forward from to help you identify and access the most effective targeted treatment for your cancer.
With our team, the entire process – from diagnosis to treatment – takes just two months. In that time, you’ll receive:
LRNA liquid biopsy to confirm whether cancer is present
PET-CT scan for precise detection and staging
Tumor DNA sequencing (550+ gene panel) to fully understand your cancer’s molecular profile
Personalized treatment recommendations – proven, leading options from around the world matched to your cancer’s unique features
This faster, more detailed process often detects cancer at an earlier stage (likely Stage 1), dramatically improving treatment success – instead of allowing the disease time to progress to a more advanced stage.
Common Questions About the LRNA Liquid Biopsy
Who is this test for?
1. Anyone who wants to know if they have active cancer, anywhere in the body, within 3 weeks – even before actual solid tumours have formed, and with uAccuracy 91.7% (95% CI: 84.7% – 98.7%), uSensitivity = 96.10% (95% CI: 90.4% – 100%), and uSpecificity = 72.7% (95% CI: 46.4% – 99.0%).
2. Cancer patients and their doctors who want the most thorough and highly accurate treatment monitoring.
3. Patients who are in remission and want to ensure they are still cancer free.

What is the fee for this test?
The LRNA Blood Test
Including a detailed and easy-to-understand results report and delivery consultation
You receive:
- Onboarding call from our team
- LRNA Blood Test
- Test Kit and blood draw
- Phase 1: Preparing the RNA for Next-Generation-Sequencing (performed by our lab team of molecular scientists and genetics specialists)
- Phase 2: NGS process looking at 20,813 genes (performed by our lab team of molecular scientists and genetics specialists)
- Written results report that you can show your doctor
- Delivery consultation
Fee: $4,500.00 CAD + GST

When will you have the results?
Within four weeks.
From the time we initiate testing to your final LRNA Test results report and delivery consultation, the entire process takes approximately four weeks.
Timeline breakdown
- Week 1: Coordination of your blood draw (LRNA Test Kit shipment and coordination of blood draw – subject to your availability and location)
- Weeks 2, 3, 4: From the time your sample arrives at our Vancouver NGS Lab, this is the time that is required for our team to process your sample and analyze your results.
- Week 4: Your results report and delivery consult for next steps
Can you have this test while on treatment?
For best results, patients should not be on treatment when the blood is drawn; a 2-3 week window is ideal.
This is usually not a concern for most patients, because most standard-of-care protocols provide a 2-3 week break between treatment rounds.
However, if you are not able to have a short treatment break, we can still provide an accurate and thorough result for you if you notify us about the treatment you are on.

How does the testing process work?
What You Can Expect
1. After you complete your intake form, our team will call you
Our team will contact you within 24 business hours (M-F) after you submit your intake form. There is an additional short form for you to fill out further details. We will ensure that any questions you have are answered and that the process is clear.
2. You receive an LRNA Test Kit by mail
Our team will arrange for an LRNA Test Kit to be shipped to you via mail.
3. A lab tech visits you at home for your blood draw
We will arrange for a laboratory technician to visit you to collect two vials of blood for testing. The laboratory technician will contact you directly to schedule the blood draw appointment at your place of residence.
4. Your blood sample is shipped to our Vancouver lab
Following your blood draw, your sample will be shipped to our next-generation sequencing lab in Vancouver, BC, Canada. This is where the first part of the process happens – including exosome separation and RNA isolation, purification, and conversion to cDNA for sequencing.
5. We analyze your LRNA Test results through our NGS technology
Our lab team then uses our state-of-the-art genetic sequencing technology to identify:
a) If molecular features involved in active cancer processes (i.e. metastasis) are present in your body
b) And, if so, to what extent and which markers are driving your cancer.
6. You receive your LRNA Test results
Within three weeks of our lab receiving your sample, your results will be presented to you in a detailed and easy-to-understand report from our team.
7. Further support and consultation is available, as needed
If your results indicate cancer activity that you were not aware of, and/or you would like to discuss treatment options based on the molecular features that we have identified through your LRNA Liquid Biopsy, our team of oncogenomic experts is standing by to help. They can provided you with a Complete Treatment Options Review – please note that additional fees apply for this.

What if the test is positive?
If your results indicate cancer activity that you were not aware of, and/or you would like to discuss treatment options based on the molecular features that we have identified through your LRNA Liquid Biopsy, our team of oncogenomic experts is standing by to help. They can provided you with a Precision Second Opinion: Complete Treatment Options Review – please note that additional fees apply for this. See below.
Additional support: Precision Second Opinion
If you would like to optimize the use of your LRNA Test results, we offer a Precision Second Opinion.
You receive:
- Collection and organization of your medical records
- Full medical records review
- Personalized research by our team of molecular scientists and genetics specialists
- Complete treatment options review (treatment recommendations proven to be more effective than your current treatment, based on the LRNA results of your cancer's molecular features)
Additional fee: $1497.00 + GST
You can add this to your test program and arrange to receive your Precision Second Opinion & Complete Treatment Options Review to coincide with your LRNA Test results.
Further support and consultation is available, as needed.

I still have questions – can I talk to someone on your team first?
Yes, absolutely. Please join one of our live, daily drop-in Zoom calls – weekdays Monday to Friday from 9:30am to 10:30am PST (12:30pm to 1:30pm PST) – where you can get immediate answers to all of your questions from cancer care specialist, Michelle Morand.
Sign up here to get the Zoom link! (Free)
Michelle Morand is co-founder of CTOAM and Liquid Biopsy Labs, and founder of Cancer: Just the Facts. She has 15+ years as an expert in cancer care advocacy and knows the ins-and-outs of the LRNA and all of our other leading-edge Next Generation Sequencing technologies.
We are happy to share our knowledge with cancer patients, loved ones, advocates, and doctors – to help empower them with the knowledge about what is possible in cancer care today...so patients can make informed cancer care choices with clarity and greater peace of mind.
See the Whole Picture
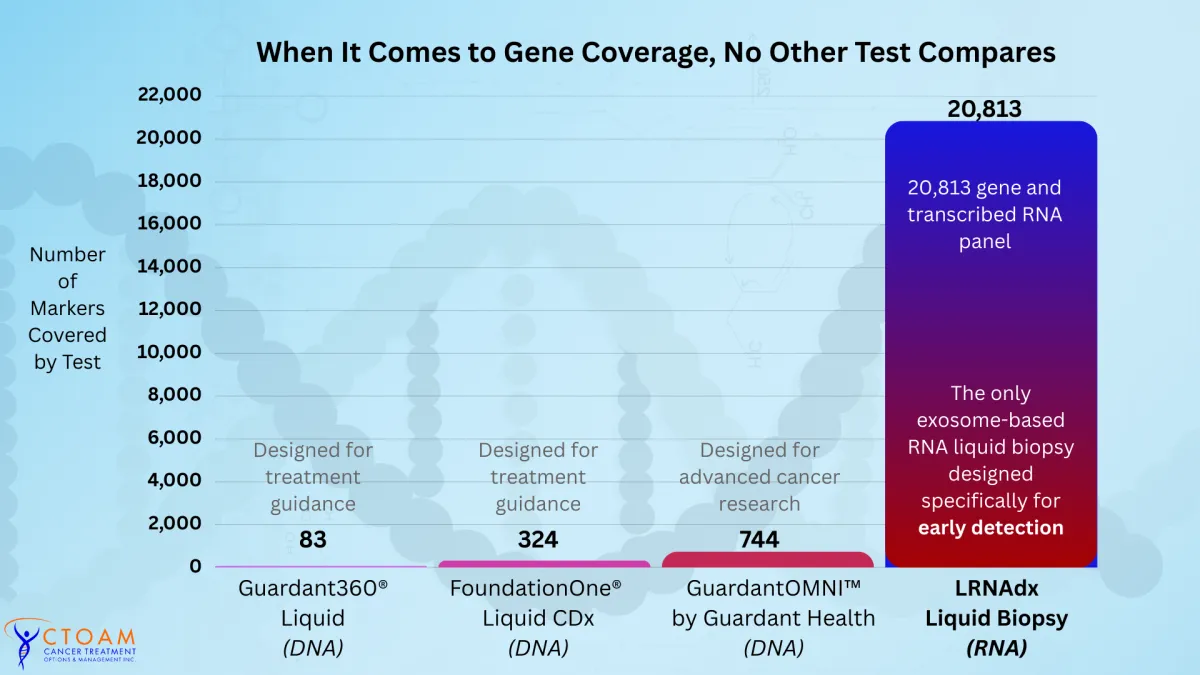
Gene Coverage that Actually Matters
When it comes to cancer testing, the difference between tests can be literally life-changing.
Most liquid biopsies on the market today are DNA-based, looking only at a small slice of cancer-related genes.
In contrast, LRNA is the first oncology test that uses epigenetic regulation to detect early cancer activity.
This means it doesn’t just capture static DNA mutations – it reveals real-time cancer activity across cancer-related molecular markers, including the latest genetic and epigenetic markers.
That’s why LRNA liquid biopsy analyzes an unprecedented 20,813 genes, compared to just 744 for the next closest test. And, unlike other tests, the LRNA does not just detect the presence or absence of a marker but it also determines the level it is expressed relative to all human tissues.
For patients, this translates into earlier detection, faster clarity, and the most comprehensive insight available into what’s really happening in the body.
Beyond DNA – Get Complete Coverage with RNA
More Genes = More Answers
The Most Accurate Diagnosis Starts with Comprehensive Gene Coverage
When it comes to cancer testing, the difference between tests can be literally life-changing.
Cancer doesn’t wait – and neither should you.
With LRNA, you can know in just three weeks whether active cancer is present, without the delays and stress of waiting months for traditional diagnostics. Because it’s the most advanced and comprehensive liquid biopsy in existence, LRNA gives you and your medical team the information needed to act quickly and confidently.
Request your LRNA test today and move forward with answers and clarity.
The Power of Early Cancer Detection – Now Made Simple
Most Cancer Patients Get Tested for Less Than 3% of Known Gene Mutations
Limited Testing Means Limited Answers
In Canada’s standard healthcare system, the genetic testing offered to cancer patients is extremely limited.
Currently, there are over 3,500 known cancer-related molecular markers and mutations – and for many of them, there are already FDA-approved treatments available or promising therapies in development.
The problem? Standard genetic testing typically checks for only 10 to 20 mutations. That’s 10 or 20 out of a possible 3,500+...as in, far too few to give most patients the complete picture they need.
By contrast, the LRNA Liquid Biopsy tests for 20,813 genes and transcribed RNA sequences – almost every known molecular feature in the human body – and looks at the latest genetic and epigenetic markers for cancer.
Issues with Standard Genetic Testing Panels:
Too small to be useful for most cancer patients
Do not provide a thorough or accurate diagnosis
Miss potential treatment options
Ignore VUSs (Variants of Unknown Significance)
Fail to account for how mutations interact with each other or influence treatment sensitivity
Often rely on tumor biopsy samples that are mishandled or degraded, leading to faulty results
The takeaway is clear: With standard genetic testing, cancer patients and their doctors do not receive the full picture (not even close!) And without that information, treatment decisions are made largely in the dark.
The LRNA Liquid Biopsy removes these blind spots by delivering a thorough genetic profile from the start – and, when paired with a comprehensive tumor DNA sequencing panel (550+ genes), these two tests give you and your medical team the complete knowledge needed to choose the most effective, targeted treatments as quickly as possible.
Simple, Non-Invasive, Personalized –
For the LRNA, One Blood Test is All You Need
Why Is Exosome Testing So Important?
We know that tumours release exosomes which carry the DNA/RNA and proteins the cancer needs to metastasize, and we know that these exosomes can also convert normal cells into tumour cells, so we need to know if they are present to ensure you get the treatment you need before cancer has a chance to progress.
This test looks to see which of those genes are over-expressed in the exosomes, meaning which genes in your blood are showing up in your blood in higher percentages than they should be when we compare your sample to normal / non-cancerous samples.
With the information this test provides we can zero in on the over-expressed genes that are highly involved in your cancer NOW and identify the level of active cancer in your body, as well as start to point towards the treatments that exist to target the most highly expressed genes.
The LRNA Test ensures that patients and doctors have more confidence in which treatment is going to be the most effective; no time wasted and no unnecessary side-effects.
It looks at 20,813 genes found in the exosomes released by tumour cells – all those identified in the widely publicized ‘human genome project’, plus a few thousand genes that have been since identified since.
Given the massive amounts of information and numerous targeted treatment options this testing identifies for each patient, it will not be long before this test is standard-of-care.
In the meantime, we are here to ensure you have the most comprehensive and accurate information from which to make your treatment decisions.
Our team has 65+ combined years in cancer care
Early cancer detection – without the clinic visit.
Step 1: Fill out your intake form
Step 2: Our team will call you within 48hrs
Step 3: We will handle everything for you*
*We will ship a testing kit to your home and arrange to have a lab tech visit you for your blood draw. Your sample is then shipped to our BC-based lab, where our team performs the LRNA testing process. You will receive your results within 3-4 weeks in a detailed, easy-to-understand report. From there, we are available for consultation on treatment options and further support, as needed (additional fees apply).
What are the Main Benefits of This Test?
Early Cancer Detection
The LRNA ensures that you catch cancer as early as possible. As much as it’s probably the last thing you want to think about if you are in remission, staying on top of any recurrence is the key to truly beating cancer.
This test is the best tool in the world for monitoring for early recurrence detection.
With the LRNA test, you can detect a recurrence before solid tumours have formed – with 95% accuracy – and many months prior to traditional methods such as Ultrasound, CT scan, MRI, and even PET/CT.
The earlier you detect cancer, the better your prognosis. If you have had cancer, you are certain to want to ensure that it doesn’t have a chance to take hold again.
Annual LRNA testing uses the most current science-based medicine and diagnostics to catch any recurrence before solid tumours can form and stay well ahead of your cancer, giving you the best chance to reduce the number of treatments you require and the amount time you spend focussing on cancer vs. living your best life.
Cancer Treatment Monitoring
The LRNA is also the easiest way for you to monitor how well your treatment is working, and to simultaneously know what treatment you will benefit from most if you need to make a change.
If your test shows you need a new treatment, our team at CTOAM is standing by to identify the best option and help advocate with your doctor / health care system to help you get it.
Screening for Cancer in Asymptomatic Cases
The LRNA is an excellent tool for peace of mind - ensuring you are cancer free far beyond the accuracy of standard of care monitoring. CA (Cancer Antigen) testing, CT scans and even PET/CT do not compare to the LRNA.
The Most Accurate Exosomal Expression Test for Early Cancer Detection
Non-Invasive Sampling: Using blood sample (plasma).
Real-Time Monitoring: Enables frequent sampling to track disease evolution and therapeutic response.
Reflects Tumor Heterogeneity: Captures cargo from different tumor clones, including metastatic and therapy-resistant cells.
High Stability: Exosomes are shed from normal and cancer cells into biological fluids, where their membranes protect RNA from degradation and enable efficient recovery.
Early Molecular Signatures: Detects cancer-specific changes before they manifest through imaging or clinical symptoms.
Potential for Personalized Medicine: Guides treatment selection and adjustment based on molecular feedback.
Patient Stories: When a Liquid RNA Test Makes All the Difference
Elena's Story

About a year after finishing treatment for colorectal cancer, Elena was finally beginning to feel like herself again. Her routine follow-ups had all come back clear, and her doctor reassured her that everything looked good. She was incredibly relieved and back to her usual daily life.
But when a close friend of hers experienced a cancer recurrence, Elena’s peace of mind was shaken.
She started to wonder: Could the same thing happen to me? What if it is, and I just don’t know it?
She brought her concerns to her doctor, but was told additional testing wasn’t needed. The scans and bloodwork showed no signs of trouble.
Still, Elena wanted to be sure. She didn’t like the feeling of concern that kept lingering in the back of her mind each day.
That’s when Elena reached out to our team. We recommended LRNA testing – an advanced molecular test that can detect signals of active cancer at the cellular level (before a tumor even forms!), long before conventional imaging picks it up.
The results were clear: residual cancer activity was still present. Something her routine follow-ups had completely missed.
With this new information, Elena decided to move forward with CTOAM’s Complete Treatment Options Review. Our scientific team used her LRNA results, along with her full medical and genetic profile, to identify the most precise, evidence-based treatment plan available – customized just for her.
We coordinated everything, so she could begin the right targeted therapy immediately. That quick action made all the difference.
Today, Elena is in remission – confident that she made the right choice to go beyond standard care.
Her story is a powerful reminder: even when routine tests say you're fine, molecular insight can reveal the truth – and save lives.
Rachel's Story

About a year after her second surgery for sarcoma, Rachel was starting to feel like life was getting back to normal. Her mid-2024 scan had come back clear, and her doctors were confident that things were on track.
But during a follow-up CT scan months later, something concerning appeared: new lesions on her shoulder and rib. A bone scan confirmed the spots, but they were dismissed as likely arthritis. She was referred to a neurosurgeon, but after a brief consultation, there was no real follow-up or plan – just a suggestion to watch and wait.
Rachel wasn’t comfortable with that – so she contacted our team.
Because sarcomas are often hard to detect with standard imaging, especially if they’re slow-growing or not PET-avid, we took a different approach. While Michelle pushed for a PET-CT scan, she also arranged an LRNA test – an advanced molecular test designed to detect signs of active cancer at the cellular level, even when scans appear normal.
The results confirmed what imaging hadn’t clearly shown: there was significant cancer activity still present in Rachel’s body.
With this new information in hand, and with the PET-CT now showing clearer signs as well, her doctor referred her to a sarcoma specialist. Within just a few days, she was scheduled for urgent, life-saving surgery to remove a spinal lesion that could have led to paralysis if left untreated.
And the impact of the LRNA test didn’t stop there.
After surgery, our team used Rachel’s molecular profile to develop a personalized, targeted treatment plan, based on the most current scientific evidence – giving her the best possible chance at a full recovery.
Today, Rachel is healing well and continuing treatment tailored to the unique biology of her cancer. She is grateful for the advancements in molecular testing and for listening to her intuition to reach out to our team.
How It Works
Catch Cancer Early – Without the Clinic Visit.
uSTEP 1
Fill Out the Intake Form
You can order the LRNA Liquid Biopsy directly from CTOAM or Liquid Biopsy Labs.
Just fill out the intake form, and our team will get things started.
You do not need a doctor to order this test.

STEP 2
Your Test Kit is Shipped to You
We will arrange for a test kit to be shipped to you and for a laboratory technician to visit you for the blood draw.
The laboratory technician will contact you directly to schedule the blood draw appointment at your home.

STEP 3
Your Blood Draw Happens
You will be visited by the lab tech at the time of your appointment.
They will follow the instructions on the LRNA Test Kit and draw two vials of blood. They will prepare the Test Kit for shipping.
STEP 4
Your Sample is Sent to our Lab
Your sample will be shipped to our next-generation sequencing lab in Vancouver, BC, Canada.
This is where the first part of the process for sequencing happens: exosome separation and RNA isolation, purification, and conversion to cDNA.

STEP 5
We Conduct the Test and Analysis
Our lab team will use our genetic sequencing technology to identify if active cancer is present.
And, if so, to what extent it is present and which genetic mutations (out of a possible 20,813 genes) are driving your cancer.

STEP 6
What Happens After Your Results
You will receive your results in a detailed report, within 3 weeks of our lab receiving your sample.
If your results show active cancer, and/or you would like to discuss treatment options, our team of oncogenomic experts is standing by to help. (Additional fees apply.)
Do You Have More Questions?
Join our Live, Daily Drop-in Zoom Calls (Free)
Want to talk with our team asap? Get your questions answered today.
Join one of our live, daily drop-in Zoom calls to get immediate answers from our co-founder & cancer care advocacy specialist, Michelle Morand.
Weekdays Monday to Friday from 9:30am to 10:30am PST (12:30pm to 1:30pm PST).
What is Liquid Biopsy Labs?
Liquid Biopsy Labs offers the world’s most accurate blood tests for early cancer detection and treatment monitoring.
Liquid Biopsy Labs and Cancer Treatment Options and Management have been leaders in the world of precision cancer medicine diagnostics, personalized research, molecular targeted treatment plan design, and cancer care advocacy for over 15 years.
That trend continues with the development of Liquid Biopsy Labs' LRNA Liquid Biopsy – most thorough and accurate tool for blood-based cancer diagnostics and treatment monitoring, as well as early detection of cancer recurrence, using RNA expression, in the world.
Liquid Biopsy Labs is the only company in the world providing this comprehensive, blood-based RNA over-expression testing of tumor-derived exosomes.
We have helped thousands of patients in Canada and around the world have a more beneficial outcome from their cancer care and live longer, healthier lives
Your Professional Care Providers
Meet our Team of Precision Cancer Medicine Experts

Michelle Morand
Co-founder, Educator and Precision Cancer Medicine Advocate

Lindsey Toane
Client-Care Coordinator and Director, and Healthcare Expert

Saima Paracha, MD
Medical Doctor, Specializing in Precision Cancer Medicine

Tara Kouhi, PhD
Research Scientist, Specializing in Precision Cancer Medicine

Noushin Moshgabadi
Research Scientist, Specializing in Precision Cancer Medicine
Our team has 65+ combined years in cancer care
Life-saving accuracy, made simple.
Step 1: Fill out your intake form
Step 2: Our team will call you within 48hrs
Step 3: We will handle everything for you*
*We will ship a testing kit to your home and arrange to have a lab tech visit you for your blood draw. Your sample is then shipped to our Vancouver-based lab, where our team performs the LRNA testing process. You will receive your results within 3-4 weeks in a detailed, easy-to-understand report. From there, we are available for consultation on treatment options and further support, as needed (additional fees apply).
Patient Testimonials
Annette F.
Stage 4 survivor, now cancer-free for 8+ years
“We have only good things to say about CTOAM. Alex was just so positive and we enjoyed our phone calls with him because after we talked to him, we were like, okay we can do this! You know, I want the person who knows the most about cancer to be managing my case. And Alex is that person. In 2015 I was diagnosed with stage 4 cancer and I didn't know if I'd be able to see my first daughter graduate highschool. Now it's 8 years later and I was able to do that! And I'm still in long-term remission."

Elke D.
Stage 3 survivor, cancer-free for 4+ years
“I’m thankful to CTOAM for all I’ve learned about cancer and have recommended you to many friends. I had a couple phone consults with Alex two years ago. As a result of his suggestions I asked for a biopsy and genetic analysis and targeted treatment. Fortunately I met the criteria for immunotherapy which has been very effective in treating my lung cancer for the past couple years. My latest scan shows no tumours and I’m off treatment now. I wish I had discovered and trusted CTOAM sooner."
Korey H.
Stage 4 survivor, now living an excellent quality of life for 5+ years
"For a lot of people, I think the route with Alex is sort of a must. I trusted him wholly right away. He shows you the science behind everything and then it’s up to you to decide what you want to do. He goes along with your choices, never forces anything on you. I wished I’d known about CTOAM a lot sooner and talked to you years ago. But thankfully it all worked out. Without Alex, I wouldn't have gotten the immunotherapy and then I'd still be on chemo. Or worse."
Learn More About RNA Sequencing
If you’d like to learn a bit more about the benefits of RNA expression testing for cancer detection and treatment identification, visit this current peer-reviewed clinical research. You can also see this 2025 article on liquid biopsy comparisons – the LRNA Liquid Biopsy is superior to all of them.
Reach Out to Us Anytime
Please reach out to us anytime if you have additional questions or would like further support. Our team of experts are here to guide you every step of the way and ensure you have access to the newest science-based technologies, treatments, and advancements in cancer care.
Do you have questions?
Use our contact form below or phone us at:
Email: admin@ctoam.com
Call toll-free: 1-833-493-5463
Call in Canada: 778-999-5463
References
Post Address and Mail
Email: admin@ctoam.com
Address
118 – 8337 Eastlake Drive, Building 3A Burnaby, British Columbia, V5A 4W2
Get In Touch
Live Assistance Hours
Mon – Fri 8:00am – 5:00pm PT
Toll-free Phone Number:
1-833-493-5463
Phone Number (Canada):
778-999-5463

Office: We serve patients worldwide. Our headquarters is in Vancouver, BC, Canada.
Call 1-833-493-5463
Email: admin@ctoam.com
Site: www.ctoam.com


